Selecting the appropriate internal standard is crucial in clinical research bioanalysis. Internal standards play an essential role in Liquid Chromatography-Mass Spectrometry (LC-MS) workflows, improving the precision and accuracy of quantitative analyses. They help researchers account for variability during sample preparation, instrument analysis, and data interpretation. Researchers face challenges like matrix effects and ion suppression, which can compromise data reliability. Thus, understanding how to effectively utilize internal standards is key to overcoming these obstacles. This guide explores the selection, timing, and practical applications of internal standards, addressing their impact on clinical research’s analytical accuracy.
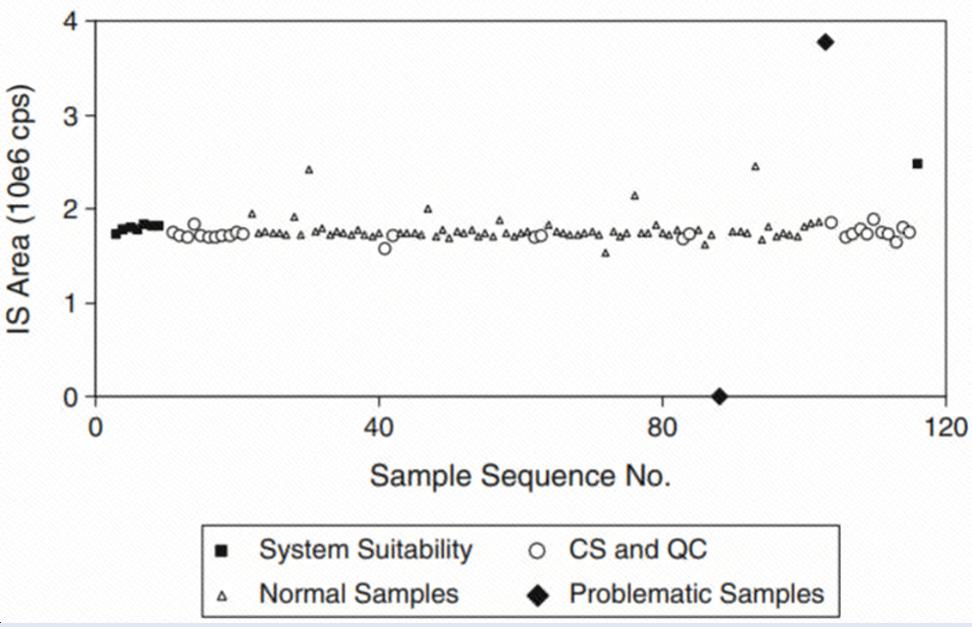
What Is an Internal Standard in Clinical Bioanalysis?
Definition and Role
An internal standard is a compound added in a constant amount to samples, calibration standards, and quality controls during LC-MS analysis. By compensating for variations in the analytical process, internal standards help ensure the consistency of experimental results. They serve as a reference point, allowing researchers to accurately quantify target analytes, thereby enhancing data reliability and reproducibility in clinical studies.
Benefits in LC-MS Workflows
Using internal standards streamlines LC-MS workflows by minimizing the impact of procedural variability. They correct for fluctuations in sample injection, changes in instrument response, and potential loss of analyte during sample preparation. By providing a stable reference, internal standards enhance the precision of compound quantitation, making them indispensable tools in complex bioanalytical processes.
Types of Internal Standards and How to Choose
Stable Isotope-Labeled (SIL-IS) Standards
Stable Isotope-Labeled Internal Standards (SIL-IS) contain isotopic atoms, making them chemically identical to the target analyte except for mass differences. SIL-IS standards are ideal for precise quantification because they closely mimic the behavior of analytes during extraction and analysis. When choosing SIL-IS, consider isotopic purity and the absence of interference with other sample components to ensure accurate results.
Structural Analogue Standards
Structural analogue standards are compounds structurally similar to the analyte but not identical. They are useful when SIL-IS availability is limited or cost-prohibitive. Though they do not perfectly mimic the analyte’s behavior, their similar chemical properties offer a practical alternative for qualitative analyses. Select analogues based on structural resemblance to the target analyte to minimize analytical deviations.
Timing of Internal Standard Addition
Pre-Extraction Addition
Adding the internal standard before sample extraction is a common approach. It compensates for losses during extraction and ensures the internal standard undergoes the same experimental conditions as the analyte. By incorporating the standard early, researchers can correct for variability from the outset of the analytical process. This strategy enhances reproducibility, minimizes variability between different runs, and provides a stronger basis for generating consistent quantitative data, especially in pharmacokinetic and toxicological studies.
Post-Extraction / Pre-Chromatography
Adding the internal standard post-extraction, but before chromatography, allows for corrections related to sample preparation, excluding the extraction step. This method may not fully account for extraction losses but circumvents issues linked to complex extraction procedures, offering a practical solution for some workflows. It is often preferred in situations where extraction introduces minimal variability, balancing practicality with analytical accuracy while reducing unnecessary complexity in sample handling protocols during routine analyses.
Post-Chromatography / Post-Column Infusion
Introducing the internal standard post-chromatography provides insights into instrument-related variation without correcting for sample preparation losses. It is useful for measuring system performance and addressing issues like ion suppression or matrix effects that predominantly occur during ionization and separation processes. This approach ensures reliable instrument monitoring, helps laboratories identify sources of variability, and is particularly valuable for method validation, troubleshooting, and long-term quality control efforts in regulated laboratory environments.
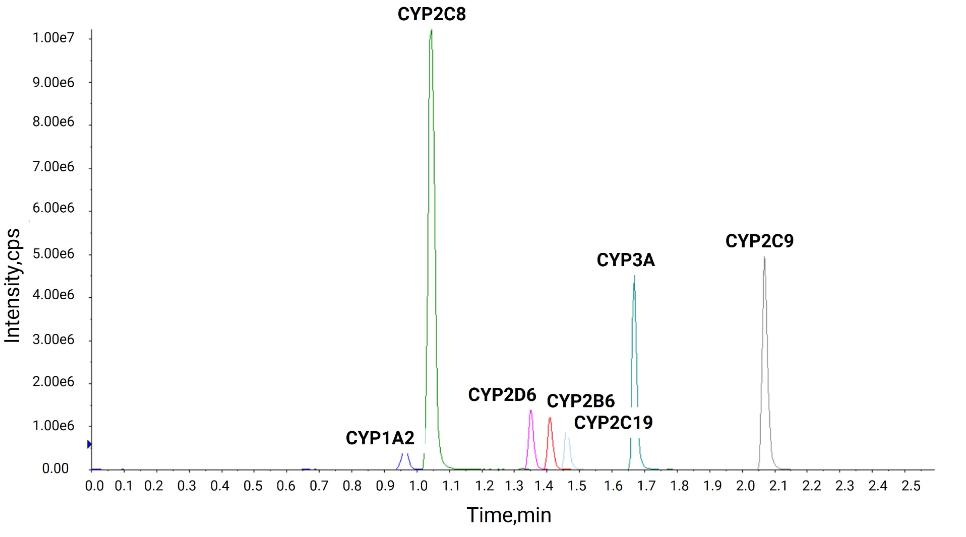
Practical Applications in Clinical Research
Ensuring Accuracy in Drug Quantitation
In drug quantitation, internal standards ensure accurate measurement of pharmaceuticals in biological matrices. By compensating for variability in LC-MS analysis, these standards allow researchers to reliably assess drug concentrations, vital for therapeutic monitoring and pharmacokinetic studies, ensuring patient safety and effective treatment. Their consistent application improves comparability across different studies, strengthens regulatory submissions, and enables precise monitoring of drug exposure over time in both preclinical and clinical trial contexts.
Addressing Matrix Effects and Ion Suppression
Matrix effects and ion suppression are common challenges in LC-MS bioanalysis. Internal standards help mitigate these issues by normalizing signals affected by matrix components, ensuring that quantitation remains reliable. Their application is critical in maintaining data integrity in complex biological matrices prevalent in clinical research. By minimizing errors caused by endogenous interferences, they improve the robustness of bioanalytical methods, supporting accurate pharmacokinetic profiling and better decision-making in drug development programs.
Conclusion
Internal standards are integral to ensuring the accuracy, reliability, and reproducibility of LC-MS bioanalytical workflows in clinical research. By selecting appropriate standards and optimizing their addition timing, researchers can effectively address method variability, matrix effects, and ion suppression issues. As clinical studies increasingly rely on precise quantitation, understanding how to implement internal standards is crucial for advancing analytical methodologies. This ensures that data can be trusted for scientific and medical decision-making, bolstering the overall integrity of clinical research.